Shanghai's BCI pioneers forge distinct paths to decode the brain
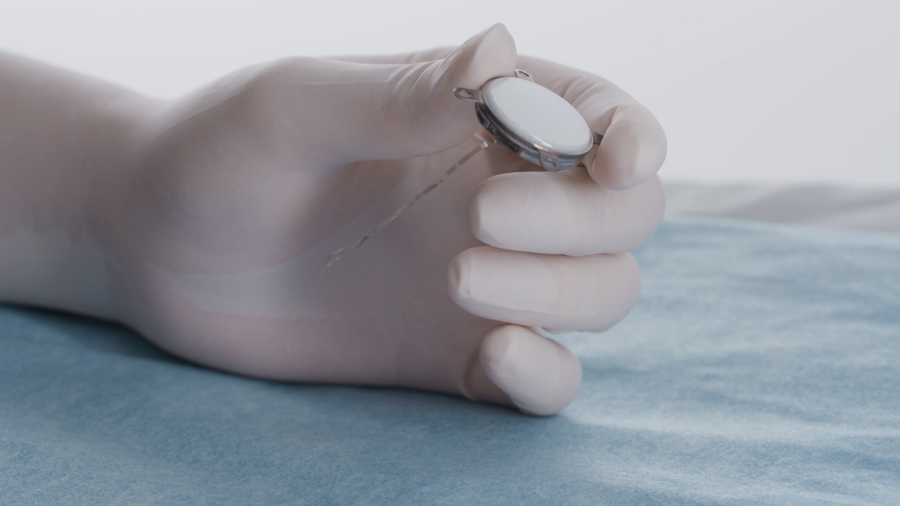
Two Shanghai-based companies, StairMed and Poda Med, have announced major progress in brain-computer interface technology, presenting innovative approaches that challenge the limits of Elon Musk's Neuralink.
StairMed has developed a revolutionary implantable wireless BCI system that has entered the National Medical Products Administration's innovative medical device fast-track review process, making it China's first invasive BCI product to access the green channel.
The company's key innovation lies in its super-flexible electrodes, which have a diameter just 1/100th of a human hair and are hundreds of times more flexible than Neuralink's products.
This design greatly reduces the risk of tissue damage and immune response after implantation, as brain tissue barely senses the electrodes.
"From the current trial results, this BCI achievement has preliminarily realized motor control function, helping patients rebuild some self-care ability," said Wu Jinsong, chief physician of neurosurgery at Huashan Hospital, which is collaborating with StairMed on prospective clinical trials.
While StairMed has optimized Musk's BCI approach through independent innovation, Poda Med is taking a completely different path that may potentially surpass Neuralink's model with ultrafast ultrasound technology.
According to Xu Kailiang, founder of Poda Med, ultrafast ultrasound is an emerging technology with frame rates reaching tens of thousands of frames per second.
Its temporal resolution is better than 10 milliseconds and spatial resolution better than 10 microns, significantly improving imaging sensitivity compared with conventional ultrasound.
Safety is another advantage of the ultrasonic BCI approach. Ultrasound probes placed outside the skull maintain resolution without being affected.
Poda Med plans to miniaturize the equipment and conduct clinical trials in two phases. First, it will place ultrasound probes outside the brain's dura mater through minimally invasive surgery. In the second phase, the probes will be positioned outside the skull to achieve truly non-invasive BCI detection.
Source: Shanghai Observer

